Once the sample is cleaned, it is transferred to an external Dewar (or another temperature-controlled container) and kept at a constant temperature. Then, a small amount of gas is introduced into the system.
The adsorbate gradually enters the evacuated sample tube. The adsorbent molecules in the sample quickly reach the solid surface of the sample, which acts as the adsorbent.
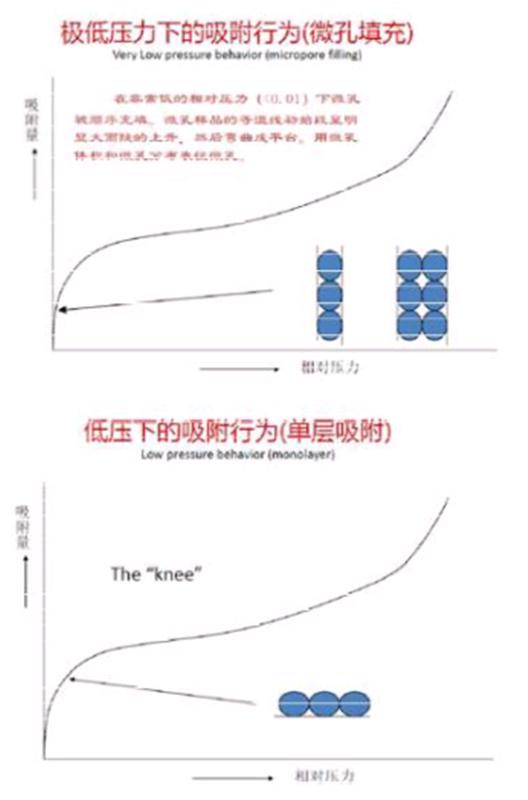
If the sample contains both micropores and mesopores, the adsorption isotherm should include several distinct stages:
1) Micropore filling (at very low relative pressure, less than 0.01):
The initial part of the isotherm shows a sharp increase, followed by a plateau. This section provides information about the micropore volume and distribution. Since the pore size is close to the diameter of gas molecules, choosing the appropriate adsorbate is essential.
2) Single-layer adsorption zone:
As more gas molecules are added, once the micropores are filled, the adsorbate forms a thin layer on the entire surface of the adsorbent. The isotherm curve shows a knee-like shape during this stage.
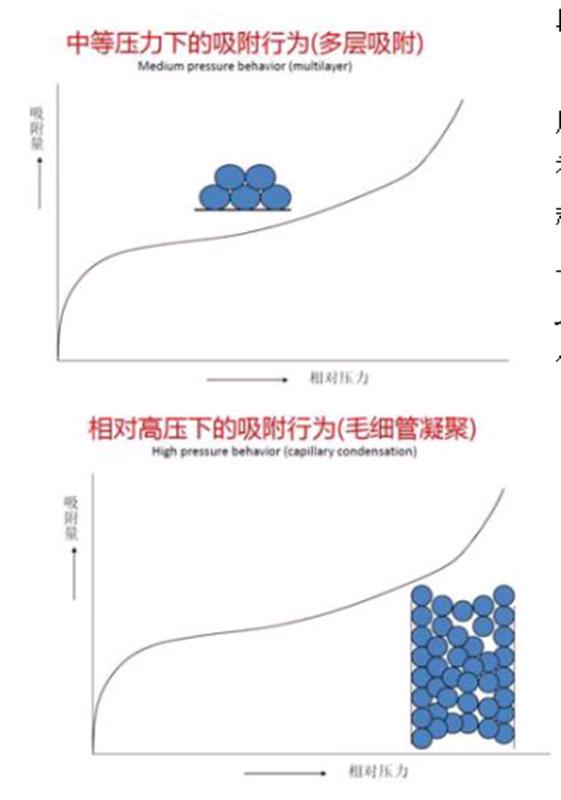
3) Multi-layer adsorption zone:
After entering the plateau region, multi-layer adsorption begins. This is where the BET theory is applied to calculate the specific surface area based on the adsorption data.
4) Capillary condensation zone:
When the relative pressure exceeds 0.4, the adsorption process is accompanied by capillary condensation. This occurs when the adsorbed gas turns into liquid within the pores as the pressure increases. The Kelvin equation describes this phenomenon, relating the equilibrium pressure to the pore size. Using methods like BJH (Barrett-Joyner-Halenda), we can calculate pore size distribution from the adsorption data.
As the adsorption reaches equilibrium and the pressure approaches saturation, the pores become completely filled with the adsorbate. Knowing the density of the adsorbate allows us to calculate the volume it occupies, and thus determine the total pore volume of the sample.
If the process is reversed by reducing the gas pressure, a desorption isotherm can be obtained. However, due to differences in adsorption and desorption mechanisms, the two isotherms rarely overlap perfectly. The hysteresis observed in the isotherm is related to the shape and structure of the pores in the solid material.
Explosion Proof Motor,Explosion Proof Servo Motor,Exproof Motors,Explosion Proof Ac Motor
Yizheng Beide Material Co., Ltd. , https://www.beidevendor.com